Title: CNS Prophylaxis Among Patients with HIV-Associated Diffuse Large B-Cell Lymphoma - A Single Center Retrospective Study
Introduction: Patients with Human Immunodeficiency Virus (HIV) are known to develop Lymphoma at a higher rate compared with the general population, with Diffuse Large B-Cell Lymphoma (DLBCL) being the most common subtype. Historically, before the advent of HIV treatment with antiretroviral therapy (ART), HIV-associated DLBCL was considered a more aggressive type of lymphoma and all patients were treated with central nervous system (CNS) prophylaxis. However, since ART has been widely adopted, there is some data to suggest that HIV-associated DLBCL among those taking ART more closely resembles de-novo DLBCL and may not need to be universally treated with CNS prophylaxis. There are no good prospective trials evaluating which patients who develop HIV-associated DLBCL while on ART should receive CNS prophylaxis. Furthermore, studies that tried to predict which DLBCL patients are at greater risk for CNS relapse explicitly excluded patients with HIV. Despite the absence of data, guidelines have shifted and current NCCN guidelines now recommend that HIV patients with DLBCL should be risk stratified as de-novo DLBCL patients with respect to CNS directed therapy. In this study, patients with HIV-associated DLBCL were retrospectively analyzed to understand the patterns of CNS prophylaxis and effects on relapse free survival.
Methods: The Electronic Medical Record was queried at a large academic medical center in New York City for patients with HIV-associated DLBCL between the years of 2012 and 2021 using ICD-10 codes. Other subtypes of Lymphoma such as Burkitt's or Plasmablastic Lymphoma were excluded. Patients who did not receive their entire treatment course within the institution were also excluded. Clinical characteristics at diagnosis, treatment type, CNS prophylaxis, and survival data was collected and analyzed.
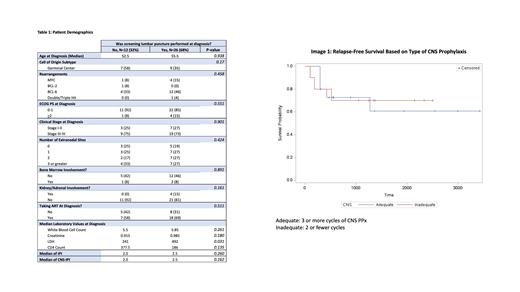
Results: A total of 38 patients were identified according to the inclusion criteria. 26 patients (68%) underwent screening lumbar punctures (LPs) for CNS disease while 12 patients (32%) did not. The two groups were not statistically different within any categories except for LDH, where the LP group had a median LDH of 492 units/L and the non-LP group had a median LDH of 241 units/L (p-value: 0.031). The two groups were not statistically different in cell of origin subtypes, stage, bone marrow involvement, kidney/adrenal involvement, the use of ART prior to diagnosis, IPI or CNS-IPI scores. In addition, among the group that had an LP done with no CNS involvement (n=25), there was no statistical difference in relapse-free survival among those who received adequate CNS prophylaxis and those who did not (p-value 0.886).
Conclusions: Among patients with HIV-associated DLBCL in a large academic medical center in New York City, there is no reliable indicator to predict which were offered screening lumbar punctures and which were not. Clinicians may be using additional measures of aggressiveness not accounted for in standard IPI and CNS-IPI scoring algorithms. Furthermore, there was no clear benefit in relapse free survival among high-risk patients who received adequate CNS prophylaxis versus those who did not in this limited sample size. Further research is needed to determine whether patients with HIV-associated DLBCL should be risk stratified similarly to de-novo DLBCL patients with respect to CNS prophylactic therapy.
Disclosures
Brody:Genentech: Research Funding; Kite Pharma: Research Funding; Merck: Research Funding; Kite-Gilead: Research Funding; SeaGen, Merck, BMS, Pharmacyclics, ADC Therapeutics, Epizyme, Genentech, Inc., Kite: Consultancy; SeaGen, Merck, BMS, Pharmacyclics, ADC Therapeutics, Epizyme, Genentech, Inc., Kite: Research Funding.